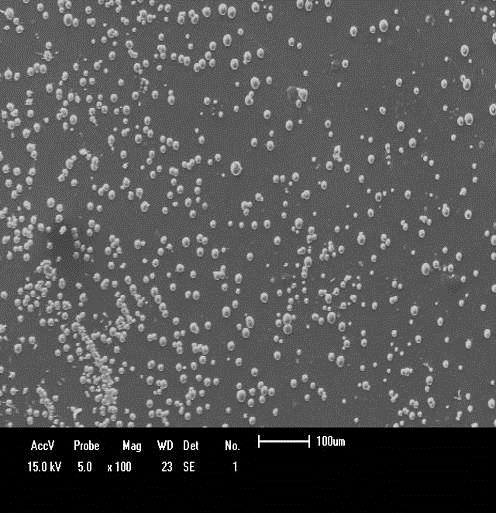
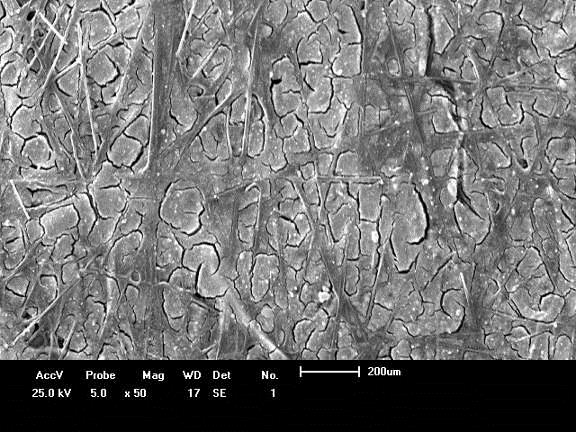
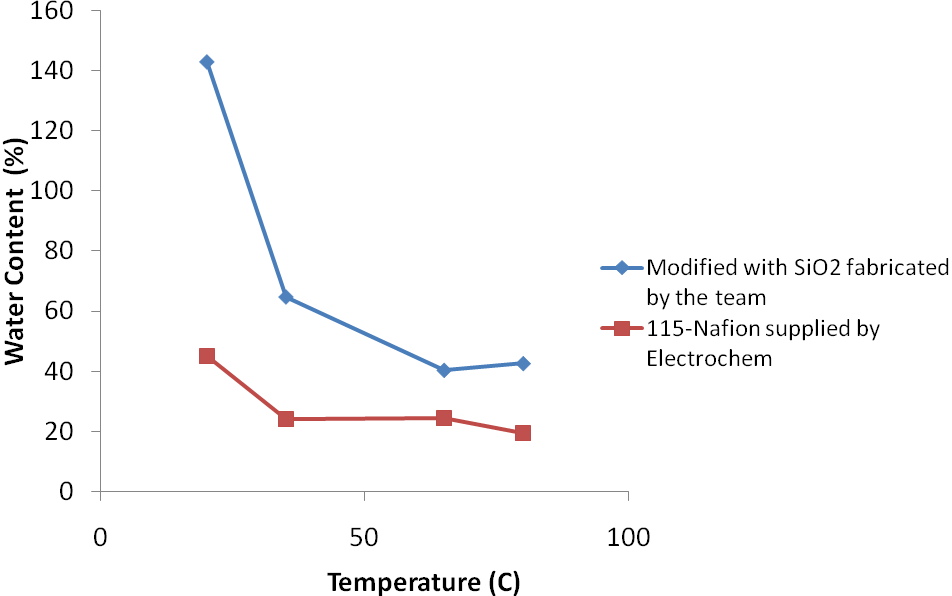
PEM Fuel Cell
Proton exchange membrane (PEM) fuel cells generate electricity and water directly through two electrochemical reactions, which take place at the interface between a proton conductive membrane and electrodes. In a PEM fuel cell, controlled hydration of the membrane is required for proper operation. The hydrogen and oxygen feed streams flow rates and directions are important in the REDOX reactions. On the other hand, the temperature of a PEM fuel cell plays an important role in the fuel cell. Accurate in situ measurements would enable improve the cell design. Our work is based on the development of the bipolar plates, proton exchange membranes, and the catalyst.